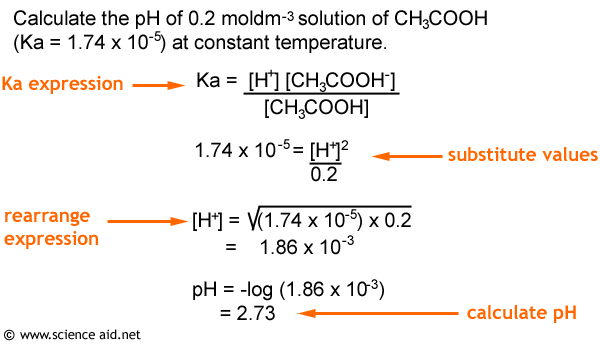
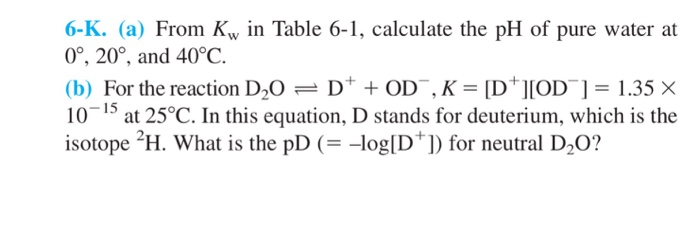
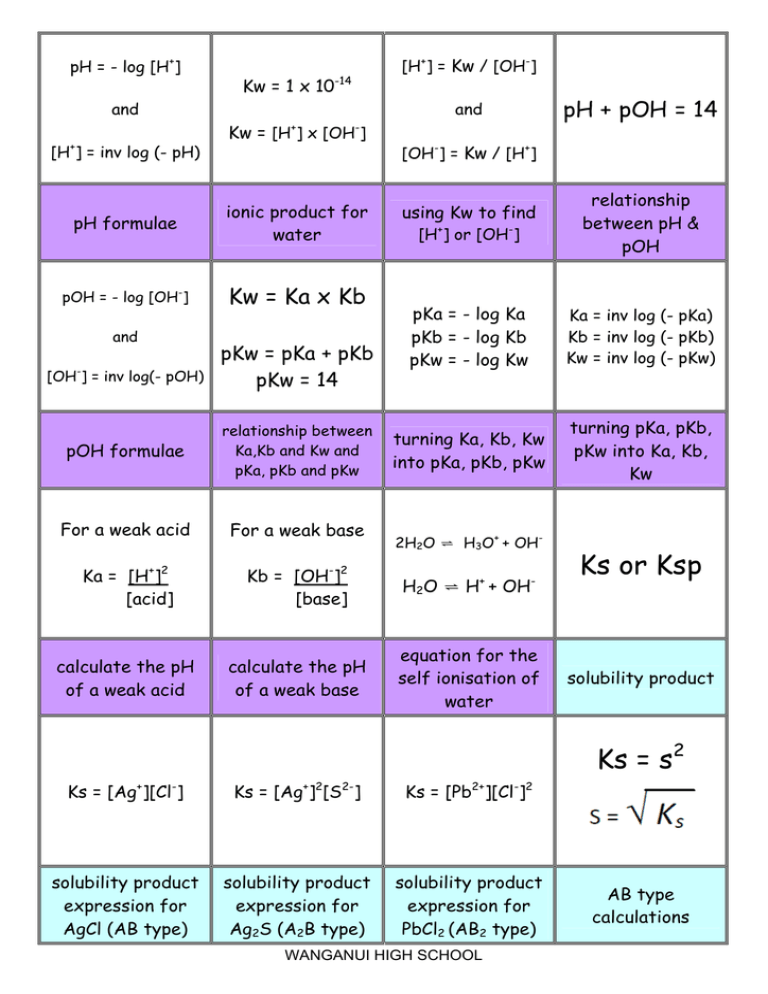
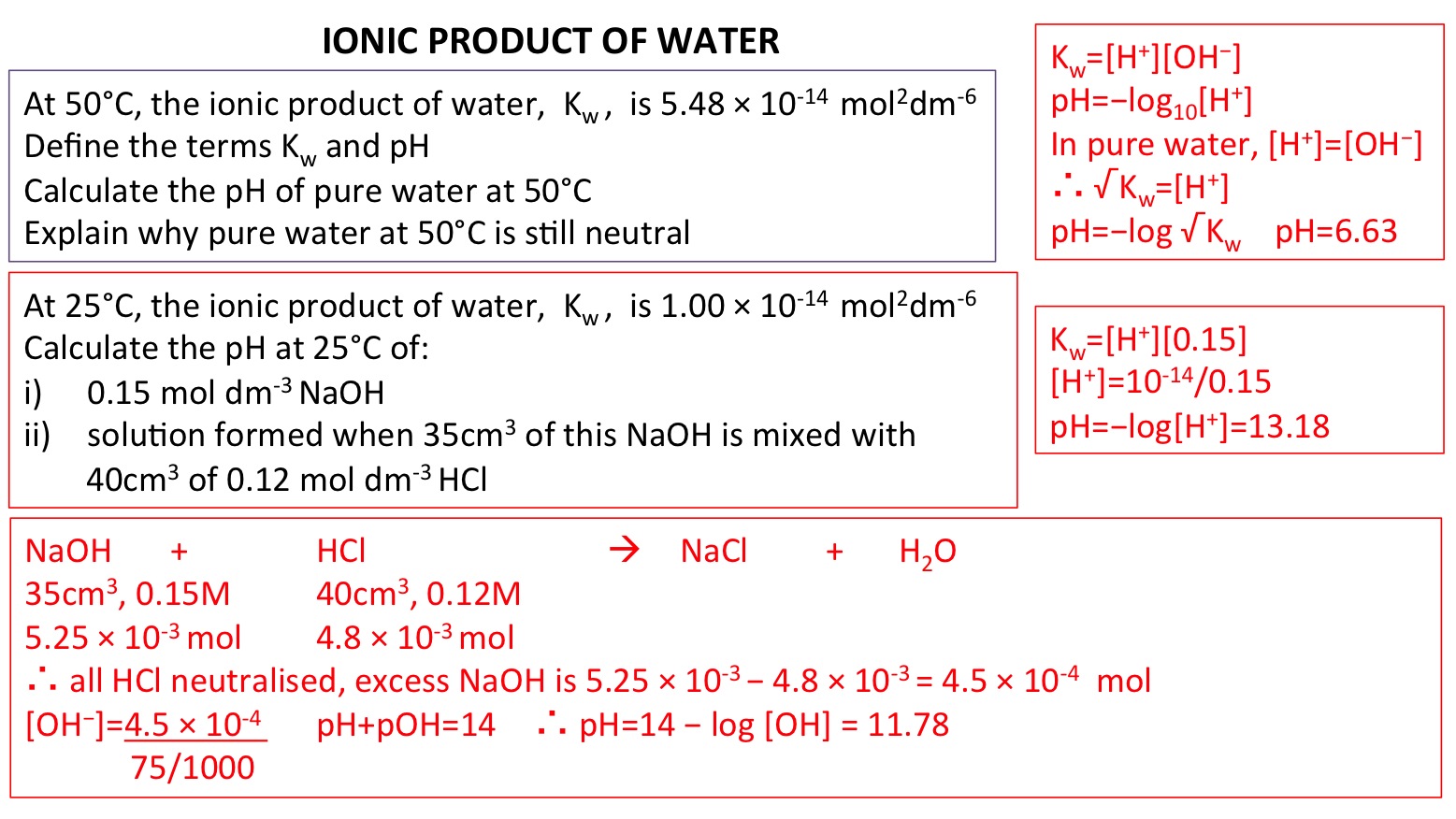
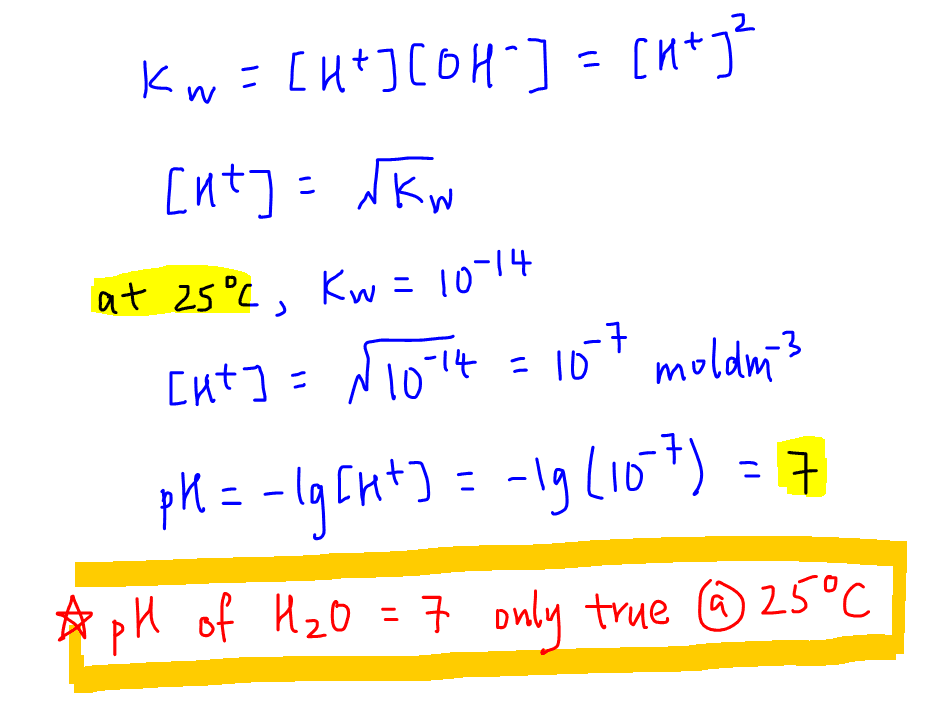The value of K_{W} is 9.55times 10^{-14} a certain temperature. Calculate the pH of water this temperature.
![PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819 PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819](https://image2.slideserve.com/5054819/slide13-l.jpg)
PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819
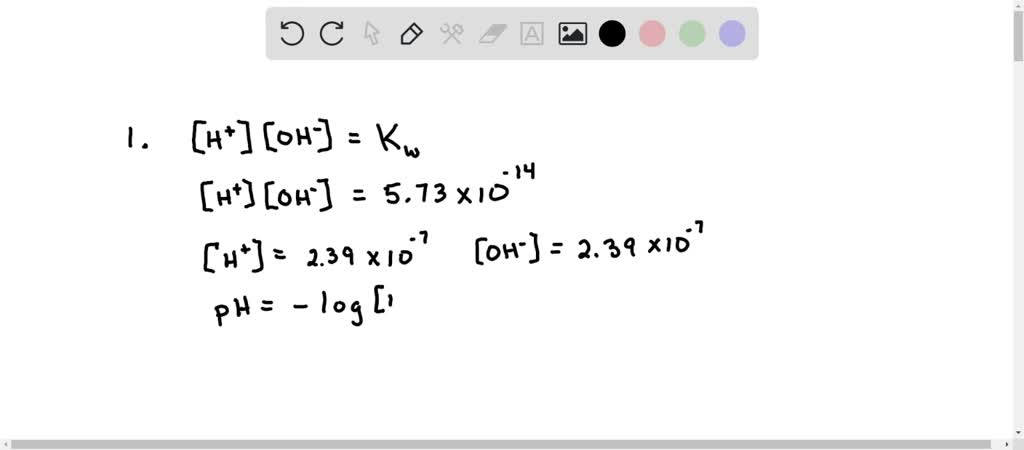
SOLVED: At 323 K, the value for Kw changes and is found to be 5.73 x 10 -14 . (i) Calculate the pH of water at this new, higher temperature. (1) (ii)
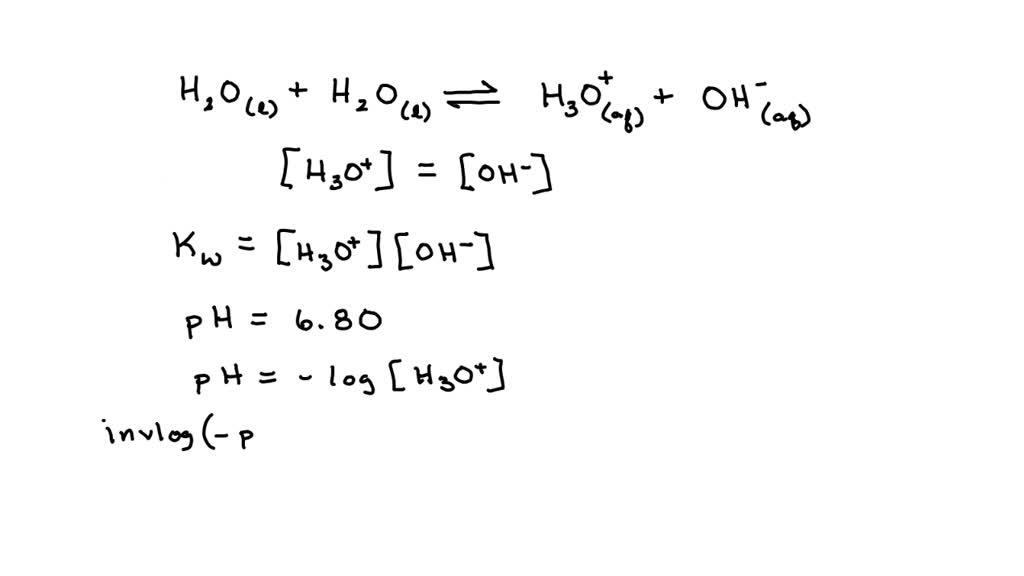
SOLVED: If the pH of pure water is 6.80 at 37 C, determine the value of KW at this temperature (it would be more relevant in medical applications that the value at 25 C).

pH from Base concentration and Ionic Product of Water calculation Workthrough - A2 Chemistry - YouTube
![SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of](https://cdn.numerade.com/ask_previews/ed96ab60-48fb-4213-8fd0-3c129172f46d_large.jpg)


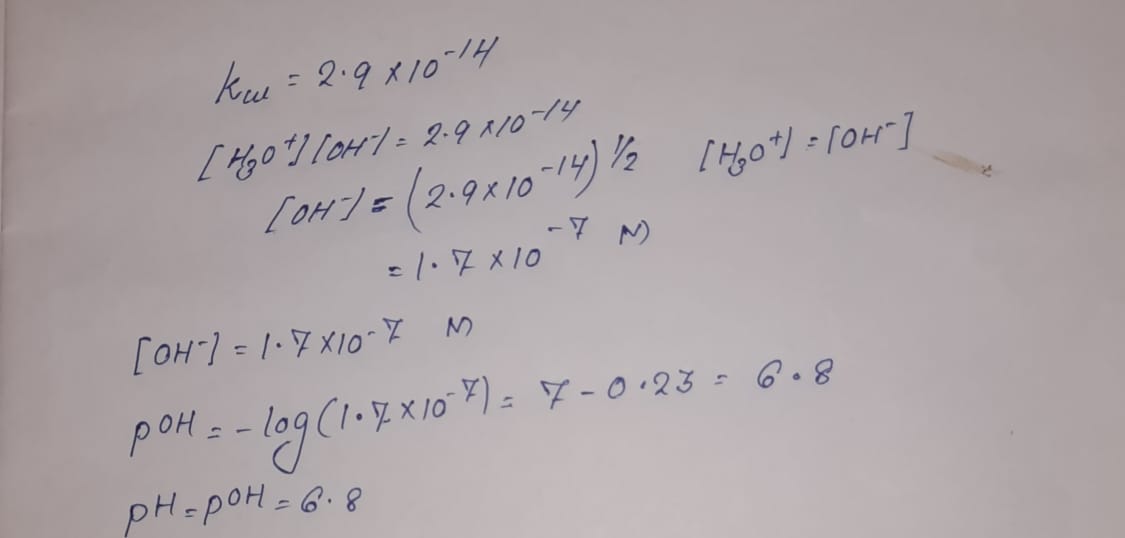


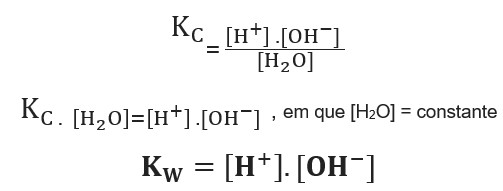

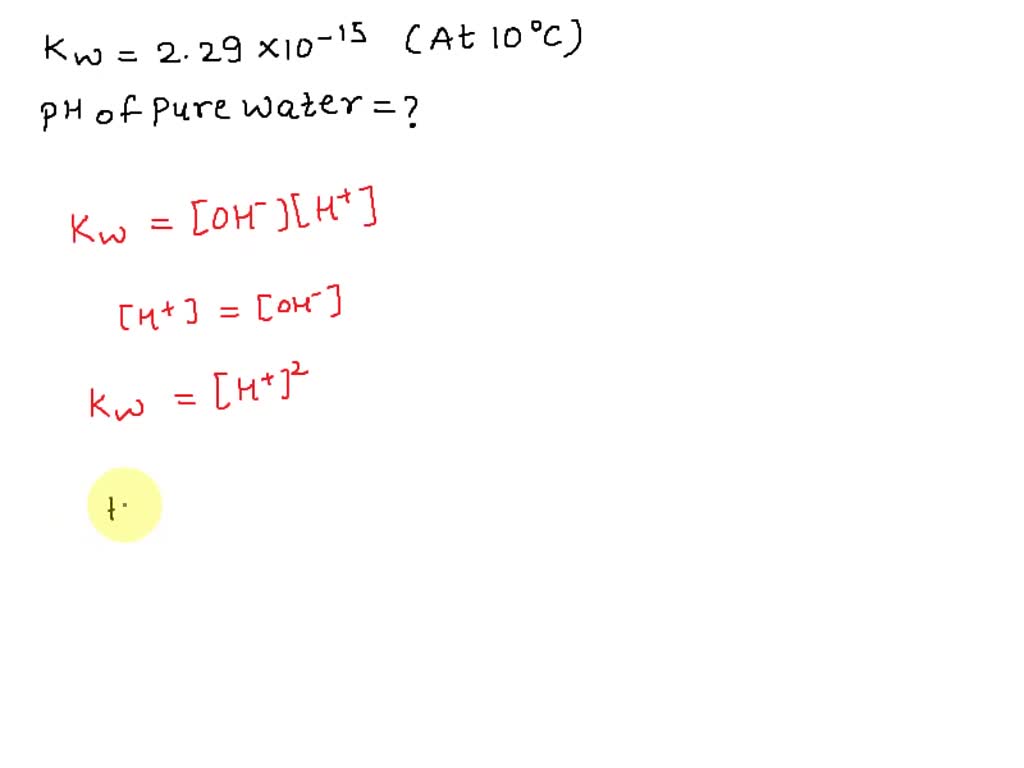

![Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube](https://i.ytimg.com/vi/IvP_PxetNUw/maxresdefault.jpg)