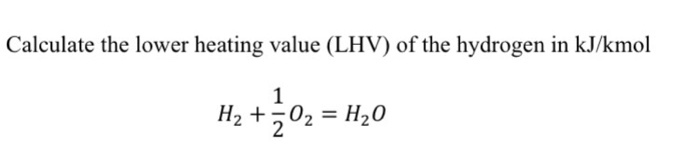Price, lower heating value (LHV) and chemical formulas of fuels and... | Download Scientific Diagram

PPT - FERTILIZER INDUSTRY LECTURE (3) Heating Value of natural gas PowerPoint Presentation - ID:1587391
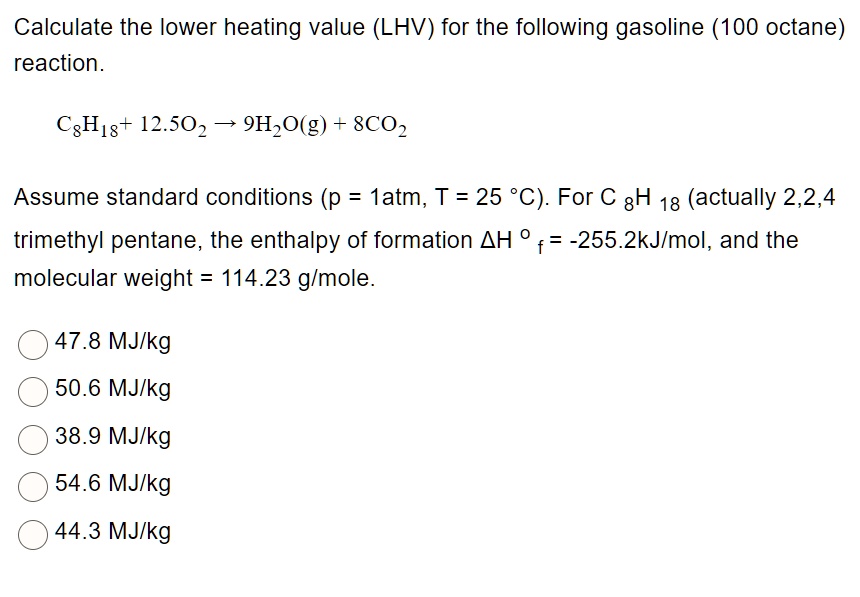
SOLVED: Calculate the lower heating value (LHV) for the following gasoline (100 octane) reaction. CgH18+ 12.5O2-> 9H2O(g)+ 8CO2 trimethyl pentane, the enthalpy of formation H , = -255.2kJ/mol, and the molecular weight =

Higher heating value of a fuel| energy | UGC NET| UPSC| NUMERICAL PROBLEM | fuel energy|heat value - YouTube
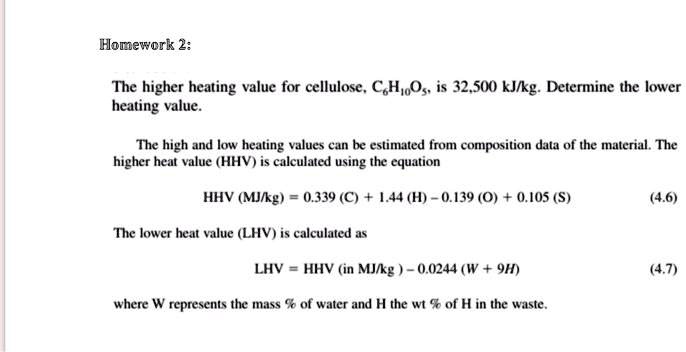
![PDF] Improving Calculation of Lower Heating Value of Waste by Data Reconciliation – Analysis and Evaluation | Semantic Scholar PDF] Improving Calculation of Lower Heating Value of Waste by Data Reconciliation – Analysis and Evaluation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/da075aa5ddc45005b9136017b718c009007cf3d7/2-Table1-1.png)
PDF] Improving Calculation of Lower Heating Value of Waste by Data Reconciliation – Analysis and Evaluation | Semantic Scholar


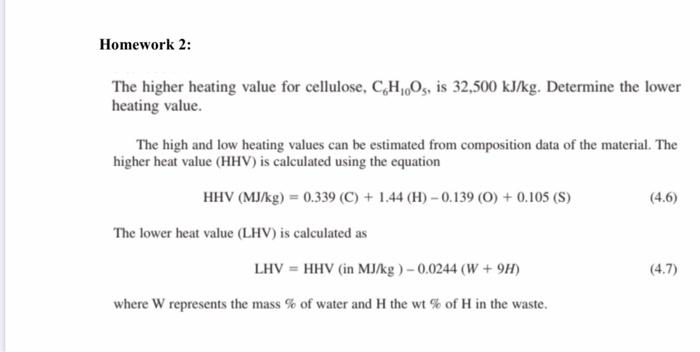
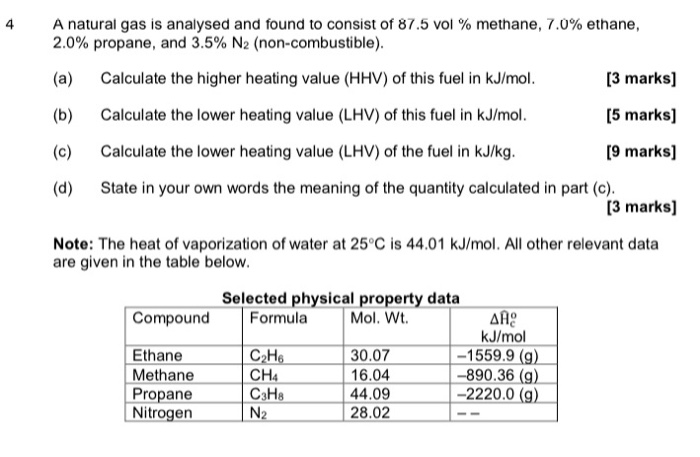

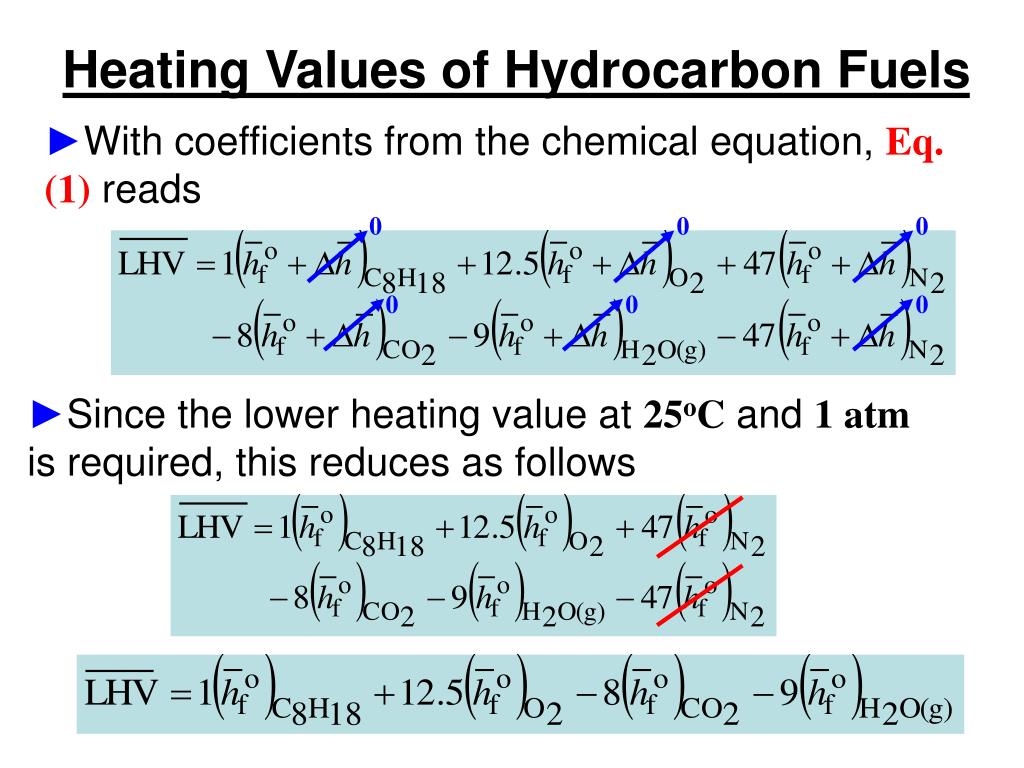



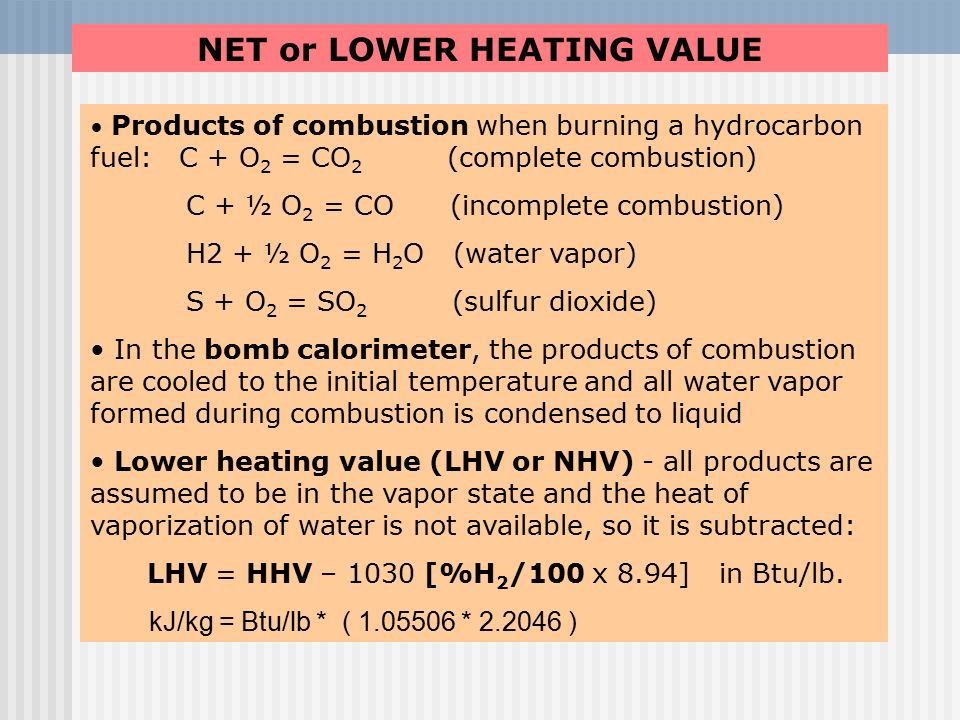

![Solved] Calculate the HHV and LHV of gaseous n-oc | SolutionInn Solved] Calculate the HHV and LHV of gaseous n-oc | SolutionInn](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1605/2/6/3/9975fae627d635821605263995759.jpg)