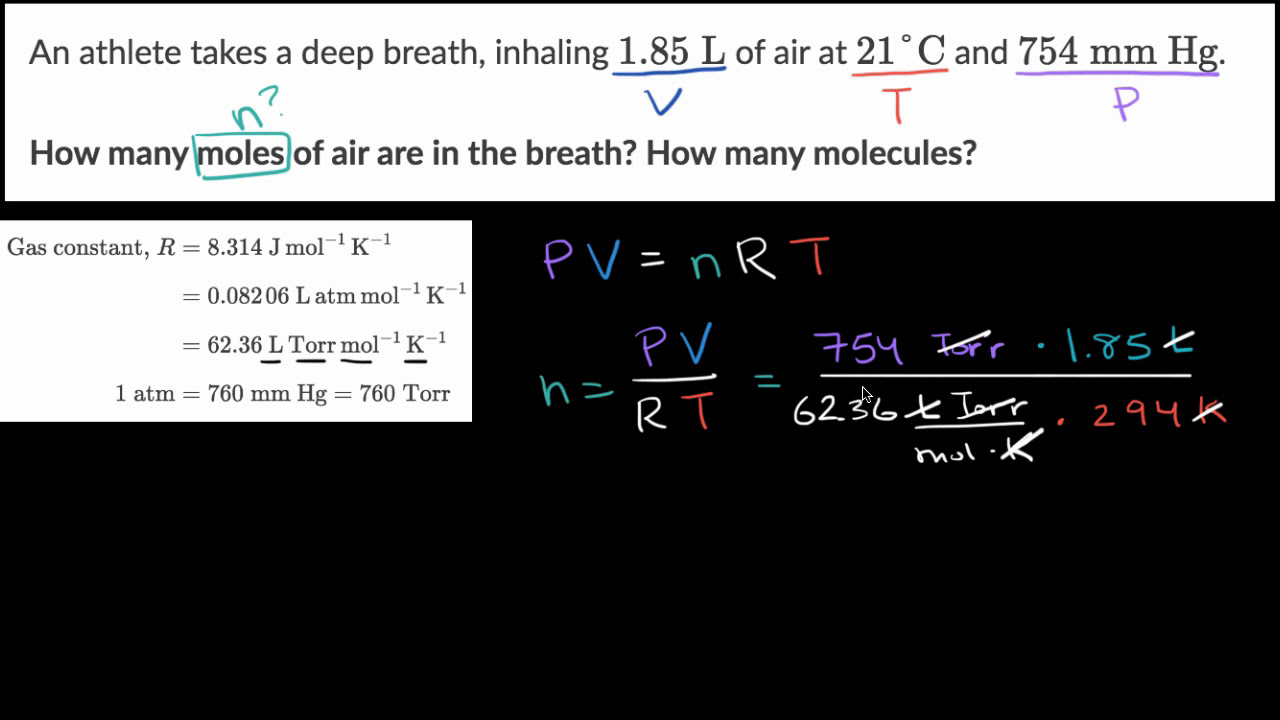
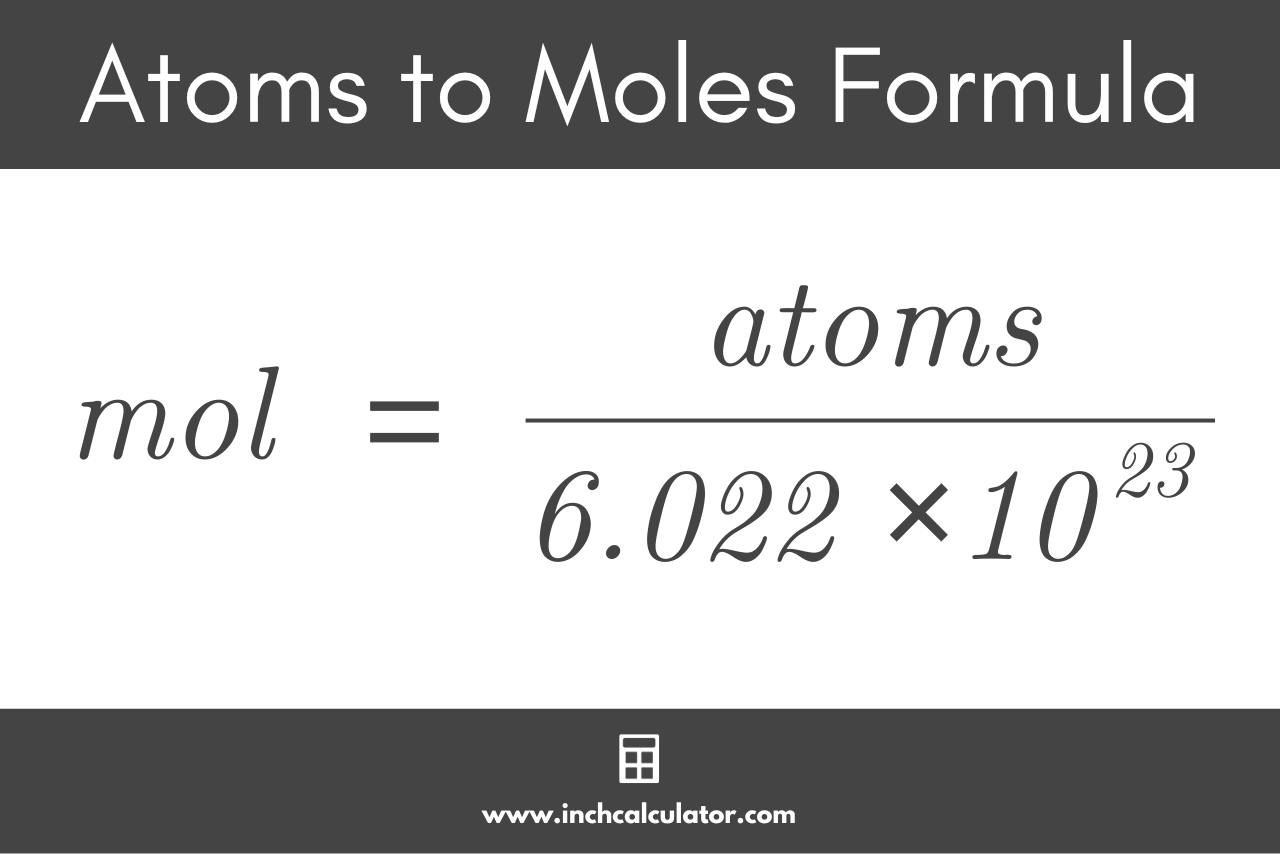
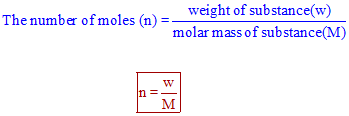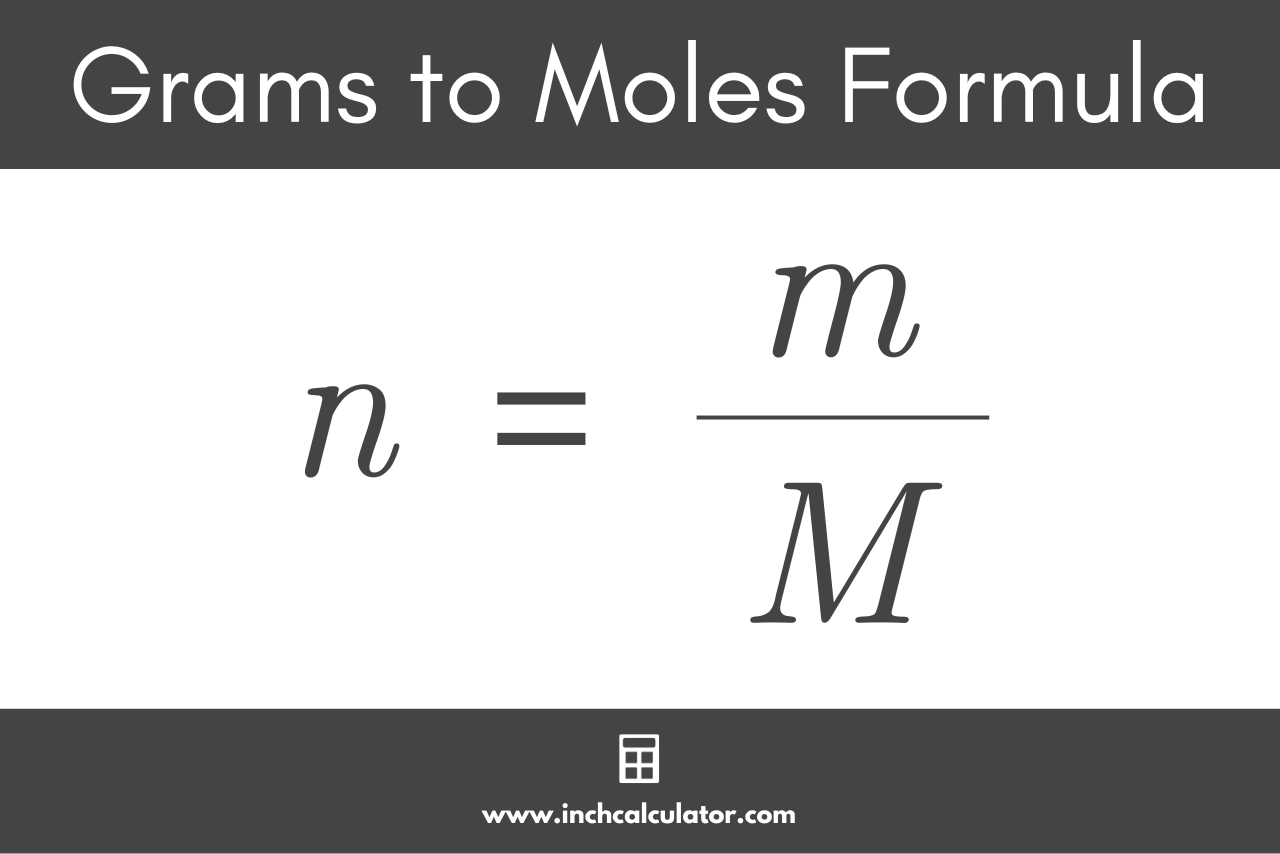Calculate the number of moles in the following:(i) 28 g of He(ii) 46 g of Na(iii) 60 g of Ca(Atomic mass of He = 4 g, Na = 23 g, Ca = 40 g) - m92pdahgg

Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g… | Chemistry education, Chemistry basics, How to calculate moles

Calculate the number of moles the following:52 g of He (finding mole from mass)12.044times 10^ {23} number of He atoms (finding mole from number of particles)
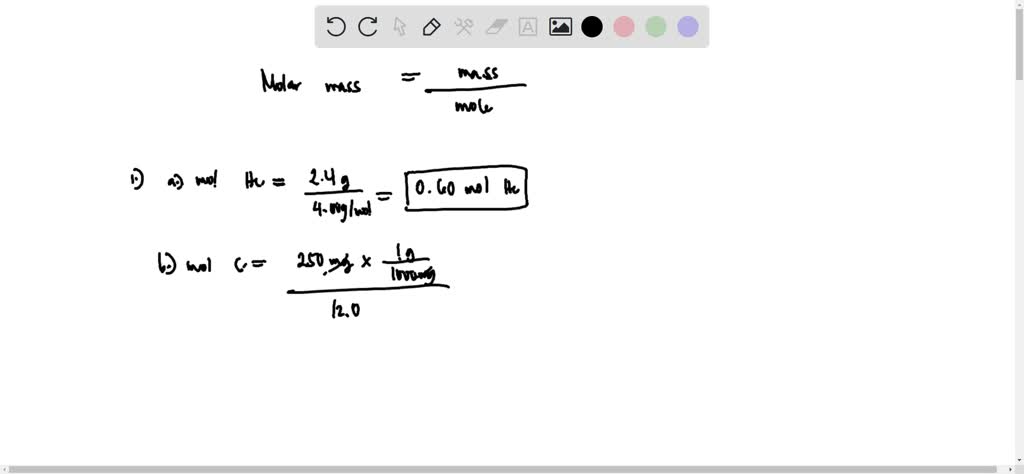
SOLVED: Q1. Calculate the number of moles of each substance in samples with the following masses: a) 2.4 g of He b) 250 mg of Carbon c) 15 g of sodium chloride